Demonstrated long-term safety profile through 96 weeks1
Nearly all individuals stayed on DESCOVY® in the DISCOVER Trial1,2
1%
of participants discontinued
DESCOVY FOR PrEP®
(n=2694)
VS
2%
of participants discontinued
FTC/TDF
(n=2693)
Side effects (adverse events) reported in ≥2% of participants1
DESCOVY(n=2694)
FTC/TDF(n=2693)
Diarrhea
5%
6%
Nausea
4%
5%
Headache
2%
2%
Fatigue
2%
3%
Abdominal pain or discomfort
2%
2%
The DESCOVY FOR PrEP safety profile at ≥144 weeks was similar to data through 96 weeks.3
Mean change in lipid values through Week 961,4-6
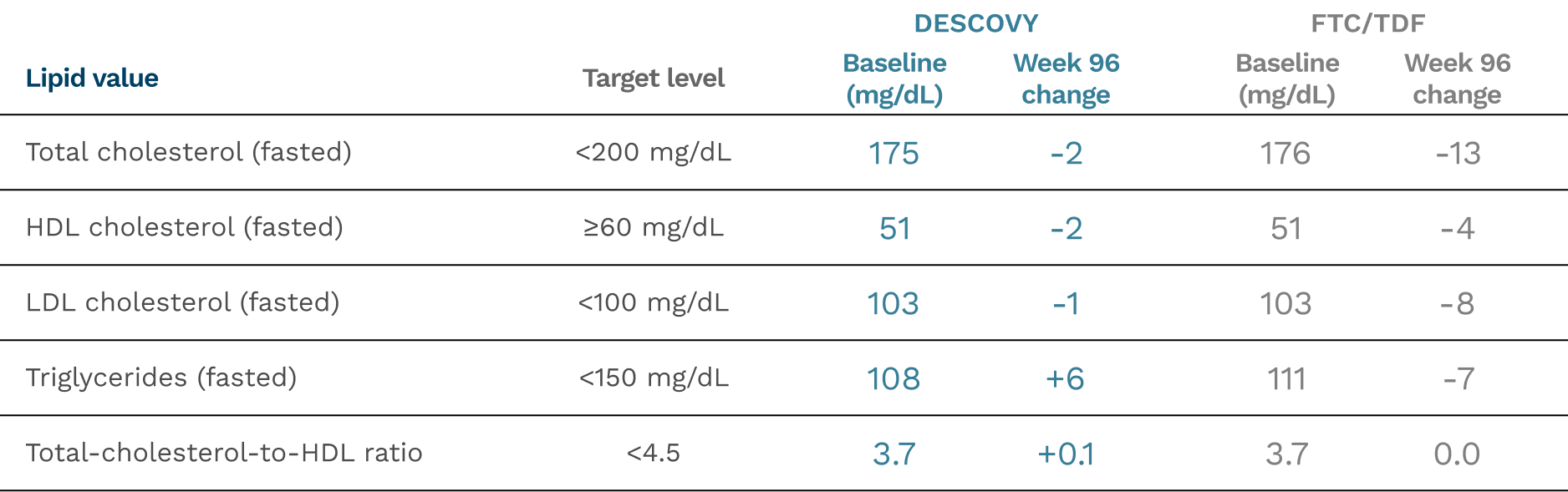
- Total-cholesterol-to-HDL ratio was similar for DESCOVY and FTC/TDF
- Decreases from baseline were seen in LDL-C, HDL-C, and total cholesterol levels in both arms
- The DESCOVY arm had increases in triglycerides vs decreases in the FTC/TDF arm; however, both were still within the target level
Mean levels of fasting lipids were mostly stable for those who were on DESCOVY over 144 weeks.3
Participants were excluded from the study if they took lipid-modifying medications at study entry or initiated the medications during the study.6
Only participants with both baseline and postbaseline fasting values were included in the above data.6
*Value reflects target for individuals assigned male at birth. For full guideline recommendations, see the 2018 AHA/ACC Multisociety Guideline on the Management of Blood Cholesterol.4
ACC=American College of Cardiology; AHA=American Heart Association; DDI=drug-drug interaction; FTC/TDF=emtricitabine/tenofovir disoproxil fumarate; HDL=high-density lipoprotein; HDL-C=high-density lipoprotein cholesterol; LDL=low-density lipoprotein; LDL-C=low-density lipoprotein cholesterol.
INDICATION & LIMITATION OF USE
DESCOVY® for HIV-1 pre-exposure prophylaxis (PrEP) is indicated in at-risk adults and adolescents (≥35 kg) to reduce the risk of sexually acquired HIV-1 infection, excluding individuals at risk from receptive vaginal sex. HIV-1–negative status must be confirmed immediately prior to initiation.
Limitation of Use: DESCOVY FOR PrEP® is not indicated in individuals at risk of HIV-1 from receptive vaginal sex because effectiveness in this population has not been evaluated.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF DESCOVY FOR PrEP® IN UNDIAGNOSED EARLY HIV‑1 INFECTION and POST-TREATMENT ACUTE EXACERBATION OF HEPATITIS B
- DESCOVY FOR PrEP must be prescribed only to individuals confirmed to be HIV negative immediately prior to initiation and at least every 3 months during use. Drug-resistant HIV-1 variants have been identified with use of emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) for HIV-1 PrEP following undetected acute HIV-1 infection. Do not initiate if signs or symptoms of acute HIV-1 infection are present unless HIV-negative status is confirmed
- Severe acute exacerbations of hepatitis B have been reported in individuals infected with hepatitis B virus (HBV) who discontinued products containing FTC and/or TDF and may occur with discontinuation of DESCOVY®. Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in individuals with HBV who discontinue DESCOVY. If appropriate, anti-hepatitis B therapy may be warranted
Contraindication
- DESCOVY FOR PrEP is contraindicated in individuals with unknown or positive HIV status
Warnings and precautions
- Comprehensive management to reduce risks:
-
- Use DESCOVY FOR PrEP to reduce the risk of HIV-1 infection as part of a comprehensive strategy that includes adherence to daily dosing and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs)
- HIV-1 risk factors: Behavioral, biological, or epidemiologic HIV-1 risk factors may include, but are not limited to: condomless sex, past or current STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network
- Reduce STI risk: Counsel on the use of STI prevention measures (e.g., consistent and correct condom use, knowledge of partner’s HIV-1 viremic status, regular testing for STIs)
- Reduce potential for drug resistance: Only prescribe DESCOVY FOR PrEP to individuals confirmed to be HIV negative immediately prior to initiation, at least every 3 months while taking DESCOVY, and upon an STI diagnosis. HIV-1 resistance substitutions may emerge in individuals with undetected HIV-1 infection who are taking only DESCOVY because DESCOVY alone is not a complete regimen for treating HIV-1
- Some HIV tests may not detect acute HIV infection. Prior to initiating DESCOVY FOR PrEP, ask individuals about potential recent exposure events. If recent (<1 month) exposures are reported or suspected, or symptoms of acute HIV infection (e.g., fever, fatigue, myalgia, skin rash) are present, confirm HIV-negative status with a test approved by the FDA for use in the diagnosis of acute HIV infection
- If HIV-1 infection is suspected or if symptoms of acute infection are present while taking DESCOVY FOR PrEP, convert the DESCOVY FOR PrEP regimen to a complete HIV treatment regimen until HIV-negative status is confirmed by a test approved by the FDA for use in the diagnosis of acute HIV infection
- Counsel on adherence: Counsel individuals to strictly adhere to daily dosing, as efficacy is strongly correlated with adherence. Some individuals, such as adolescents, may benefit from more frequent visits and counseling
- New onset or worsening renal impairment: Postmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with tenofovir alafenamide (TAF)-containing products. Do not initiate DESCOVY in individuals with estimated creatinine clearance (CrCl) <30 mL/min. Individuals with impaired renal function and/or taking nephrotoxic agents (including NSAIDs) are at increased risk of renal-related adverse reactions. Discontinue DESCOVY in individuals who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Monitor renal function in all individuals (see Dosage and Administration section)
- Lactic acidosis and severe hepatomegaly with steatosis: Fatal cases have been reported with the use of nucleoside analogs, including FTC and TDF. Discontinue use if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity develop, including hepatomegaly and steatosis in the absence of marked transaminase elevations
Adverse reactions
- Most common adverse reactions (≥2%) in the DESCOVY FOR PrEP clinical trial were diarrhea, nausea, headache, fatigue, and abdominal pain
Drug interactions
- Prescribing information: Consult the full Prescribing Information for DESCOVY for more information, warnings, and potentially significant drug interactions, including clinical comments
- Metabolism: Drugs that inhibit P-gp can increase the concentrations of TAF, a component of DESCOVY. Drugs that induce P-gp can decrease the concentrations of TAF, which may lead to loss of efficacy
- Drugs affecting renal function: Coadministration of DESCOVY with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of FTC and tenofovir and the risk of adverse reactions
Dosage and administration
- Dosage: One tablet (emtricitabine 200 mg/tenofovir alafenamide 25 mg) taken once daily with or without food
- HIV screening: Test for HIV-1 infection immediately prior to initiating, at least every 3 months during use, and upon diagnosis of an STI (see Warnings and Precautions section)
- HBV screening: Test for HBV infection prior to or when initiating DESCOVY
- Renal impairment and monitoring: Not recommended in individuals with CrCl <30 mL/min. Prior to or when initiating DESCOVY, and during use on a clinically appropriate schedule, assess serum creatinine, CrCl, urine glucose, and urine protein in all individuals. In individuals with chronic kidney disease, assess serum phosphorus
Please see full Prescribing Information for DESCOVY FOR PrEP, including BOXED WARNING.
References: 1. Ogbuagu O, Ruane PJ, Podzamczer D, et al; the DISCOVER study team. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet HIV. 2021;8(7):e397-e407. doi:10.1016/S2352-3018(21)00071-0 2. Descovy. Package insert. Gilead Sciences, Inc.; 2022. 3. Wohl DA, Spinner CD, Flamm J, et al. HIV-1 infection kinetics, drug resistance, and long-term safety of pre-exposure prophylaxis with emtricitabine plus tenofovir alafenamide (DISCOVER): week 144 open-label extension of a randomised, controlled, phase 3 trial. Lancet HIV. 2024;11(8):e508-e521. 4. About cholesterol. Centers for Disease Control. Published May 15, 2024. Accessed July 10, 2024. https://www.cdc.gov/cholesterol/about/index.html 5. Millán J, Pinto X, Munoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757-765. 6. Data on file. Gilead Sciences, Inc.

